 |
 | |
| Species: |
Cynomolgus monkey |
| Strain/breeder: |
Purpose breed |
| Sex: |
Female |
| Age: |
2 years |
| Study type: |
Intermittent iv infusion via port catheter system (PCS) |
| Treatment: |
Dose without organ toxicity |
| Animal status: |
Scheduled kill (study week 40) |
| Clinical findings: |
7 months before necropsy: port catheter system defect, catheter visible at skin wound, repeated treatment with antibiotics, 2 months before necropsy: explantation of PCS necessary |
| Organ(s): |
Kidney |
Macroscopic
finding(s): |
Size decreased, massive, unilateral |
| Staining: |
H&E |
|
 |
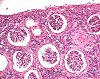
Fig. 1 (116k)
|
|
Abstract
Septicaemic kidney alterations after intermittent intravenous infusion using a port-catheter system in a cynomolgus monkey toxicity study
J. HELLMANN
Institute of Toxicology, Merck KGaA, Frankfurter Str. 250, 64271 Darmstadt, Germany
Key words: port catheter infusion technique, cynomolgus monkey, septicaemia
To support clinical trials, 44 cynomolgus monkeys received intermittent short-term infusions (1 h, 3x per week) for a period of 39 weeks. In order to avoid stress, perivenous inflammation, or thrombosis a vascular access port system (VAP) was used. This comprised a port device in the back with a catheter to the femoral vein, both implanted entirely subcutaneously with access via a minipump and needle.
Five deaths occurred within the first 10 weeks of treatment. Embolic purulent inflammation and bacteraemia were seen in multiple organs, associated with exhaustion of the immune system. The kidneys exhibited various lesions, from acute purulent-embolic nephritis and disseminated intravascular coagulation with acute bilateral cortical necrosis to chronically shrunken kidneys after 39 weeks. Bacteriology revealed that staphylococcus aureus accounted for most of the lesions observed with E. coli contributing in some cases. In conclusion, intermittent infusions bear the risk that bacteria might proliferate inside the implanted ports or catheters if residual amounts of administered solution in the devices are contaminated during skin penetration despite careful precautions.
case index | << previous case | next case >>
|
 |